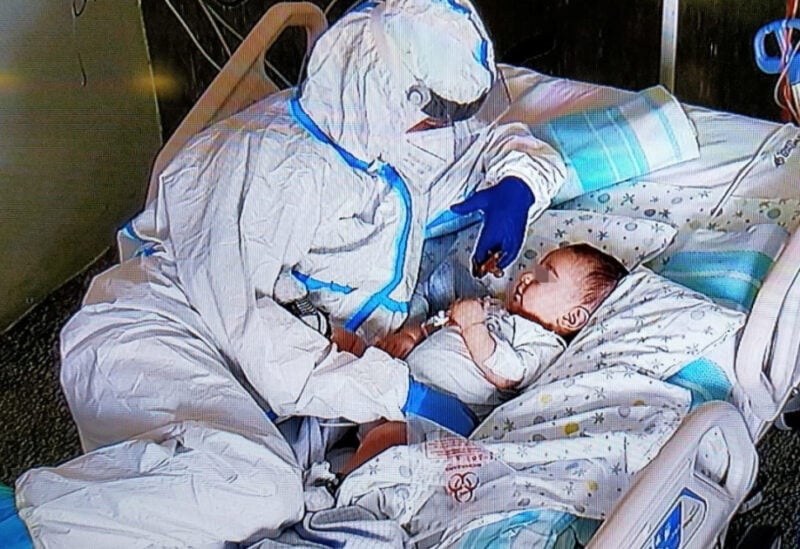
A nurse comforts a seven-month-old baby at the Salesi Hospital following an operation after his parents were unable to visit due to coronavirus restrictions, in Ancona, Italy, March 22, 2021.
BioNTech expects results by September from trials testing the coronavirus vaccine that it and Pfizer have developed in babies as young as 6 months old, German magazine Spiegel quoted the company’s CEO as saying.
“In July, the first results could be available for the 5 to 12 year olds, in September for the younger children,” BioNTech Chief Executive Ugur Sahin told Spiegel. He also said it takes about 4 to 6 weeks to assess the data.
“If all goes well, as soon as the data is evaluated, we will be able to submit the application for approval of the vaccine for all children in the respective age group in different countries,” Sahin added.
This month, BioNTech and Pfizer asked U.S. regulators to approve emergency use of their vaccine for adolescents aged 12 to 15.
A trial published at the end of March found the companies’ coronavirus vaccine was safe, effective and produces robust antibody responses in adolescents.
The Pfizer/BioNTech 2-dose vaccine is already authorized for use in those aged 16 and above.
Young people are less likely to suffer severe cases of COVID-19 and more likely to have asymptomatic infection, allowing them to unwittingly transmit the coronavirus to others.